Viral O-Glycoproteomics Service
Based on a high-resolution mass spectrometry platform and highly selective glycopeptide enrichment technology, MtoZ Biolabs has launched the viral O-glycoproteomics service to perform systematic analysis of O-glycoproteins in virus-derived samples. This service covers glycopeptide enrichment, O-glycosylation site identification, glycan structure characterization, and relative quantitative analysis, enabling accurate revelation of glycosylation composition features and distribution patterns. Through precise data processing and database comparison, researchers can obtain high-confidence information on glycopeptide sites, glycan type distribution, and modification ratios, providing reliable data support for viral protein function studies and host interaction analysis.
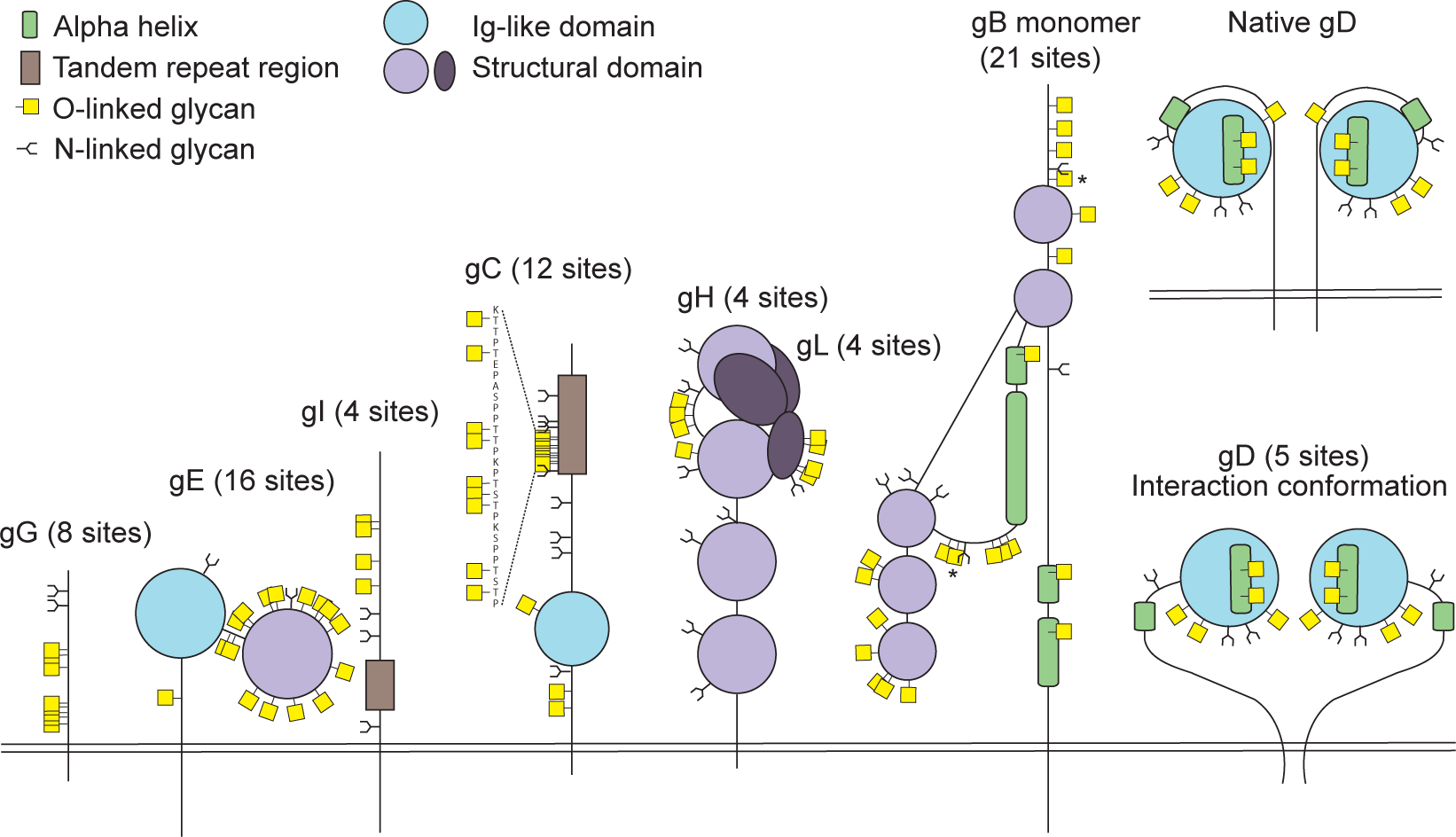
Bagdonaite, I. et al. PLoS Pathog, 2015.
Figure 1. Identified O-Linked Glycosylation Sites on Herpes Simplex Virus Type 1 Envelope Glycoproteins
Overview
Viral O-glycoproteomics is an omics research field that focuses on analyzing O-linked glycosylation modifications in viral proteins. O-glycosylation is a common post-translational modification that typically occurs on serine (Ser) or threonine (Thr) residues, influencing viral protein folding, immune recognition, receptor binding, and infectivity. By systematically identifying O-glycosylation sites and structural features of viral proteins, this research provides key molecular information for understanding viral infection processes and host interactions.
Analysis Workflow
1. Sample Preparation and Protein Extraction
Total proteins are extracted from viral or infected samples and enzymatically digested to obtain peptide mixtures.
2. O-Glycopeptide Enrichment
O-glycosylated peptides are enriched using lectin affinity or HILIC methods to enhance detection sensitivity.
3. Mass Spectrometry Detection and Data Acquisition
High-resolution LC-MS/MS platforms are used to detect glycopeptide signals and acquire fragment spectra.
4. Data Analysis and Structural Identification
Databases and computational algorithms are applied to identify glycosylation sites and glycan structures.
5. Result Integration and Report Generation
Modification features are statistically analyzed and visualized, followed by the generation of a comprehensive report.
Sample Submission Suggestions
1. Sample Type
Purified viral samples, infected cell lysates, or viral protein extracts are accepted; lyophilized powder, solution, or tissue samples are also supported.
2. Sample Storage
Samples should be stored at −80°C for long-term preservation or at −20°C for short-term use; repeated freeze-thaw cycles should be avoided to prevent protein degradation and glycan loss.
3. Sample Transportation
Sealed and moisture-proof packaging is recommended; liquid samples should be shipped under cold-chain conditions, while lyophilized samples can be sent at room temperature for short periods but should be protected from heat and direct light exposure.
Service Advantages
1. High-Sensitivity Detection Platform
Utilizing the Orbitrap LC-MS/MS system enables precise qualitative and quantitative analysis of low-abundance O-glycopeptides.
2. Multilayer Enrichment Strategies
Combining lectin affinity, HILIC, and chemical derivatization methods enhances O-glycosylation coverage and detection depth.
3. Standardized Analytical Workflow
A rigorous sample preparation and quality control system ensures data stability and reproducibility.
4. Customizable Research Solutions
Tailored analytical and data interpretation services are provided according to viral type and research objectives.
Applications
1. Viral Glycoprotein Characterization
Systematic analysis of O-glycosylation features on viral surface glycoproteins reveals their structural diversity.
2. Host Interaction Studies
The viral O-glycoproteomics service can be used to evaluate the binding characteristics between viral glycoproteins and host cell receptors or adhesion molecules.
3. Evaluation of Novel Antiviral Strategies
By analyzing the effects of drug treatment or genetic intervention on viral O-glycosylation levels, potential inhibitory mechanisms can be assessed.
4. Antibody Binding Characterization
The viral O-glycoproteomics service enables evaluation of how O-glycosylation influences viral antigen epitope exposure and antibody recognition.
FAQ
Q1: Can Different O-Glycan Types on Viral Glycoproteins Be Analyzed?
A1: Yes. By combining high-resolution mass spectrometry with glycan database matching, MtoZ Biolabs can distinguish core structural types (such as Core 1, Core 2, etc.) and determine their modification patterns and distribution characteristics.
Q2: Does the Host Expression System Affect O-Glycosylation Results?
A2: Yes. Mammalian, insect, and plant cells have different glycosyltransferase systems, leading to variations in glycan types, substituents, and modification ratios. Therefore, it is recommended to specify the host source when submitting samples.
Q3: What Types of Viruses Are Suitable for O-Glycosylation Studies?
A3: This service is applicable to various enveloped and secretory glycoprotein viruses, including coronaviruses, influenza viruses, Ebola viruses, and herpesviruses.








