Arginylation Analysis Service
Arginylation is the covalent addition of arginine residues to proteins, mediated by arginyltransferases, and is involved in regulating protein turnover, cytoskeletal organization, stress responses, and cellular signaling. Despite its biological importance, the dynamic and context-dependent nature of arginylation makes it challenging to detect and interpret. MtoZ Biolabs provides a dedicated Arginylation Analysis Service that includes both targeted analysis of specific proteins and proteome-wide profiling.
1. Target Protein Arginylation Analysis
For studies focused on individual proteins, MtoZ Biolabs offers targeted analysis to identify arginylated sites, quantify modification levels, and assess arginylation-dependent effects such as altered stability or degradation. High-resolution LC–MS/MS, PRM/MRM quantification, immunoaffinity enrichment, and optional NMR-based structural evaluation can be applied as needed.
2. Arginylation Proteomics
For broader discovery-driven investigations, our arginylation proteomics workflow enables large-scale mapping of arginylated proteins across complex biological samples. Through high-throughput LC–MS/MS acquisition and integrated bioinformatics, we provide comprehensive identification, quantitative comparison across conditions, and pathway-level interpretation to uncover functional networks regulated by arginylation.
What is Arginylation?
Arginylation is a unique post-translational modification in which an arginine residue is enzymatically added to proteins, typically at exposed N-terminal or midchain sites. The modification is catalyzed by arginyltransferases (ATE1 in mammals), which transfer arginine from tRNA to target proteins.
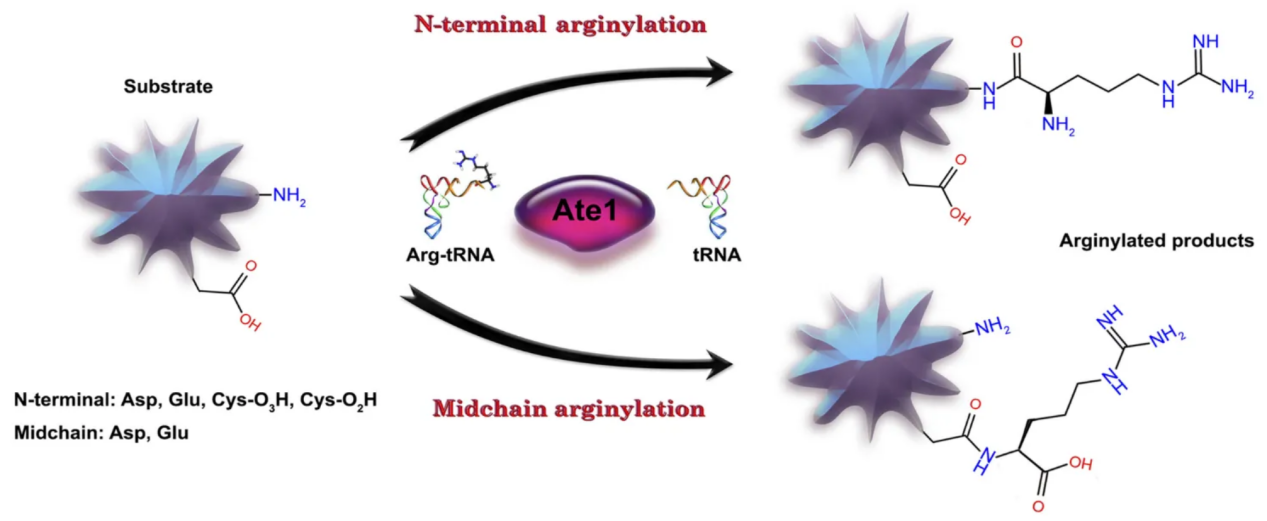
Galiano, M. R. et al. J Neurochem. 2016.
Figure 1. Reactions (Schematic) During N-Terminal and Middle-Chain Arginylation of Proteins
Arginylation directly impacts protein stability by linking to the N-end rule pathway of protein degradation. Through this pathway, proteins carrying arginylated residues may be selectively marked for ubiquitination and subsequent proteasomal degradation. This modification also plays critical roles in regulating actin cytoskeleton remodeling, cell adhesion, stress adaptation, and cardiovascular function. Studies have shown that defective arginylation is associated with embryonic lethality, cardiac malformations, and tumorigenesis. The functional diversity of arginylation lies in its ability to modulate both protein half-life and signaling functions, making it a crucial regulatory mechanism in health and disease.
Analysis Workflow
1. Sample Preparation
Proteins are extracted, quantified, and enzymatically digested under optimized conditions to preserve arginylation.
2. Enrichment Strategies
Immunoaffinity purification using arginylation-specific antibodies or chemical enrichment is employed to selectively capture modified peptides.
3. Mass Spectrometry Detection
High-resolution LC-MS/MS platforms, including Orbitrap systems, are used for accurate site-level identification. Targeted quantification is enabled through PRM or MRM workflows.
4. Bioinformatics and Data Interpretation
Customized computational pipelines provide modification site validation, quantitative analysis, pathway annotation, and functional mapping of arginylation events.
Sample Submission Suggestions
1. Sample Types
Cell lysates, tissue extracts, biofluids (plasma, serum, CSF), or purified proteins.
2. Amount Required
At least 100 µg of total protein.
3. Storage and Shipping
Samples should be snap-frozen in liquid nitrogen and shipped on dry ice to maintain integrity.
*Note: For other sample types, please contact us in advance for tailored preparation guidance.
Service Advantages
✅ Precision: Site-level identification with high-resolution mass spectrometry.
✅ Sensitivity: Optimized enrichment enables detection of low-abundance arginylated peptides.
✅ Comprehensive Coverage: Full workflow from sample preparation to pathway-level interpretation.
✅ Customization: Tailored experimental designs to meet diverse project needs.
✅ Expert Support: Professional interpretation of results with scientific and technical guidance.
Applications
Arginylation Analysis Service has wide applications across basic and translational research:
1. Cell Signaling Regulation
Profiling arginylation-dependent modulation of protein stability and signaling dynamics.
2. Cytoskeletal Biology
Investigating the role of arginylation in actin remodeling, cell motility, and adhesion.
3. Stress and Adaptation Mechanisms
Understanding arginylation in oxidative stress responses and adaptive signaling.
4. Cardiovascular and Developmental Biology
Exploring arginylation-related pathways in embryogenesis, cardiac development, and tissue remodeling.
5. Disease Mechanism Studies
Identifying links between aberrant arginylation and cancer, neurodegenerative diseases, or metabolic disorders.
6. Drug Discovery and Target Validation
Evaluating arginylation as a potential therapeutic target and biomarker in precision medicine.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
With advanced analytical platforms, customizable workflows, and expert-driven support, MtoZ Biolabs is your trusted partner in unlocking the full potential of arginylation proteomics research. Contact us today to discuss your project and request a custom proposal.