S-Glutathionylation Analysis Service
S-glutathionylation represents a critical redox-dependent post-translational modification in which glutathione forms a reversible disulfide bond with protein cysteine residues. This dynamic process functions not only as a protective mechanism against irreversible oxidative damage but also as a regulatory signal influencing protein folding, enzymatic activity, and cellular stress responses.
MtoZ Biolabs provides a comprehensive S-Glutathionylation Analysis Service that integrates multiple complementary technologies to capture both the breadth and specificity of this modification. Our workflows employ high-resolution mass spectrometry for site-specific mapping, Western blotting for validation and semi-quantitative detection, and biotin-switch assays for selective enrichment of modified proteins. These approaches provide detailed insights into both specific proteins of interest and large-scale modification patterns across complex biological systems.
1. Target Protein S-glutathionylation Analysis
For focused investigations, MtoZ Biolabs provides targeted S-glutathionylation analysis, where specific proteins of interest are studied for modification sites and modification levels. This approach is particularly useful for studying the functional implications of S-glutathionylation on signaling proteins, redox-sensitive enzymes, and other key regulators involved in oxidative stress responses.
2. S-glutathionylation Proteomics
For a broader, discovery-driven approach, MtoZ Biolabs offers S-glutathionylation proteomics to profile modification events across the entire proteome. This method combines high-throughput sample processing, biotin-switch enrichment, and quantitative LC-MS/MS analysis to map glutathionylation sites globally. By comparing modification patterns under various experimental conditions, we gain a system-wide understanding of how S-glutathionylation regulates cellular processes such as metabolism, apoptosis, and signal transduction. This service also includes downstream bioinformatics analysis for pathway enrichment and protein interaction network mapping.
What is S-Glutathionylation?
S-glutathionylation arises when reduced glutathione (GSH) forms a reversible disulfide bond with the sulfhydryl group of cysteine residues in proteins. Glutathione itself is a tripeptide composed of glutamate, cysteine, and glycine, and it represents the most abundant low-molecular-weight thiol in cells. This modification is facilitated either through enzymatic catalysis by glutaredoxins or through non-enzymatic reactions triggered by reactive oxygen and nitrogen species. By temporarily "capping" reactive cysteines, S-glutathionylation prevents irreversible oxidation to sulfinic or sulfonic acids, thereby preserving protein function under oxidative stress conditions.
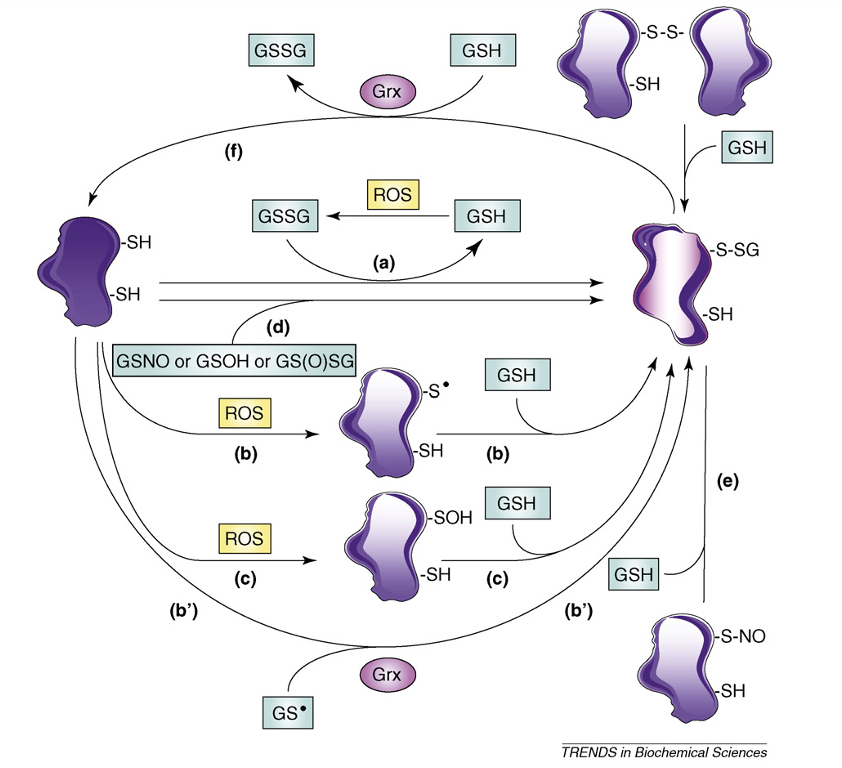
Dalle-Donne, I. et al. Trends Biochem Sci. 2009.
Figure 1. Main Mechanisms of Protein S-glutathionylation and Deglutathionylation
Beyond its protective capacity, S-glutathionylation also acts as a regulatory switch in cellular signaling. It can alter protein conformation, modulate catalytic activity, affect protein–protein interactions, and control subcellular localization. These regulatory effects have been observed in enzymes of central metabolism, cytoskeletal proteins, ion channels, transcription factors, and signaling kinases. As such, S-glutathionylation contributes to the fine-tuning of pathways that govern cell survival, apoptosis, immune activation, and metabolic adaptation.
Given its dual role as both a protective antioxidant mechanism and a signaling mediator, S-glutathionylation has emerged as a key biomarker of oxidative stress and a functional regulator of disease progression. Accurate identification and quantification of this modification are therefore essential for deciphering molecular mechanisms, discovering diagnostic markers, and developing novel therapeutic strategies.
Analysis Workflow

Sample Submission Suggestions
1. Sample Types
Cells, tissues, serum, plasma, or purified proteins are suitable. For other sample types, please contact us for customized guidance.
2. Protein Quantity
A minimum of 1–2 mg of total protein for global analysis; smaller amounts may suffice for targeted studies.
3. Handling Conditions
Avoid strong oxidants or reductants that may alter glutathionylation levels.
4. Storage
Flash-freeze samples in liquid nitrogen and store at –80°C.
5. Shipping
Ship on dry ice to maintain sample stability.
6. Replicates
Biological replicates are recommended for robust statistical interpretation.
Service Advantages
☑️High Sensitivity: Advanced enrichment methods combined with Orbitrap LC-MS/MS enable detection of low-abundance modifications.
☑️Customizable Workflows: Flexible protocols adapted to diverse sample types and research goals.
☑️Expert Team: Scientists with extensive experience in redox proteomics and PTM analysis provide reliable data interpretation.
☑️Efficient Turnaround: Streamlined workflows ensure timely delivery of results.
Applications
S-Glutathionylation analysis supports a broad range of research applications:
1. Redox Biology
Understanding how glutathionylation regulates protein activity under oxidative stress.
2. Signal Transduction
Elucidating redox-dependent modulation of receptors, kinases, and transcription factors.
3. Disease Mechanisms
Investigating the role of abnormal glutathionylation in cardiovascular disease, diabetes, cancer, and neurodegenerative disorders.
4. Biomarker Discovery
Identifying glutathionylated proteins as indicators of oxidative stress or disease progression.
5. Drug Development
Evaluating the impact of therapeutics on cellular redox balance and protein glutathionylation.
FAQ
Q: How can we avoid loss or reversal of S-glutathionylation during sample preparation?
Lyse immediately in a denaturing buffer and cap free thiols with NEM or IAA (avoid DTT/β-ME). Aliquot, snap-freeze at −80 °C, and ship on dry ice to preserve native S-glutathionylation.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment
4. Data Analysis, Preprocessing, and Estimation
5. Bioinformatics Analysis
6. Raw Data Files
Our S-Glutathionylation Analysis Service is designed to provide more rapid, high-throughput, and cost-effective analysis, with exceptional data quality and minimal sample consumption. Free project evaluation, welcome to learn more details.








