Collagen Lysine Hydroxylation and Cross-linking Analysis Service
Collagen lysine hydroxylation and subsequent cross-linking shape the mechanical properties and biological functions of connective tissues. These processes determine the structural stability of collagen fibrils and regulate interactions with surrounding matrix components. MtoZ Biolabs provides a specialized Collagen Lysine Hydroxylation and Cross-linking Analysis Service to help researchers and industry partners systematically investigate the structural and functional consequences of collagen modifications.
Our comprehensive service portfolio includes:
💠Site-specific Identification
Mass spectrometry-based peptide analysis allows unambiguous detection of hydroxylysine residues and mapping of cross-linking sites across collagen chains.
💠Quantitative and Dynamic Profiling
Label-free quantification and targeted MS approaches, including PRM and MRM, monitor changes in hydroxylation and cross-linking across biological conditions or treatment groups.
💠Cross-link Structural Analysis
We identify and characterize mature cross-links such as hydroxylysyl pyridinoline (HP) and lysyl pyridinoline (LP), using mass spectrometry and chemical derivatization, complemented by NMR for three-dimensional structural assessment.
💠Tissue-level Localization
Immunohistochemistry enables visualization of hydroxylated and cross-linked collagen within tissue sections, linking molecular events to histological context.
💠Bioinformatics Integration
Comprehensive pathway mapping and network analysis provide functional interpretation, connecting collagen modifications to biological processes such as tissue remodeling, fibrosis, and disease progression.
Background
Collagen is the most abundant structural protein in mammals and represents the primary component of connective tissues, including bone, cartilage, tendon, and skin. Its unique triple-helical architecture provides tensile strength and resilience, while its post-translational modifications fine-tune structural stability and biological functionality. Proper maturation of collagen molecules is therefore essential for tissue integrity and homeostasis.
Among these modifications, lysine hydroxylation is a critical enzymatic reaction mediated by lysyl hydroxylases. In this process, specific lysine residues located within the collagen helical domain or telopeptide regions are hydroxylated to form hydroxylysine. This modification not only stabilizes the triple helix through additional hydrogen bonding but also creates reactive sites for further biochemical processing. Hydroxylysine residues serve as substrates for glycosylation and as essential precursors for covalent cross-links, thereby influencing collagen solubility, fibril organization, and interactions with matrix proteins. Dysregulation of lysine hydroxylation has been associated with connective tissue disorders, bone fragility syndromes, and fibrotic pathologies.
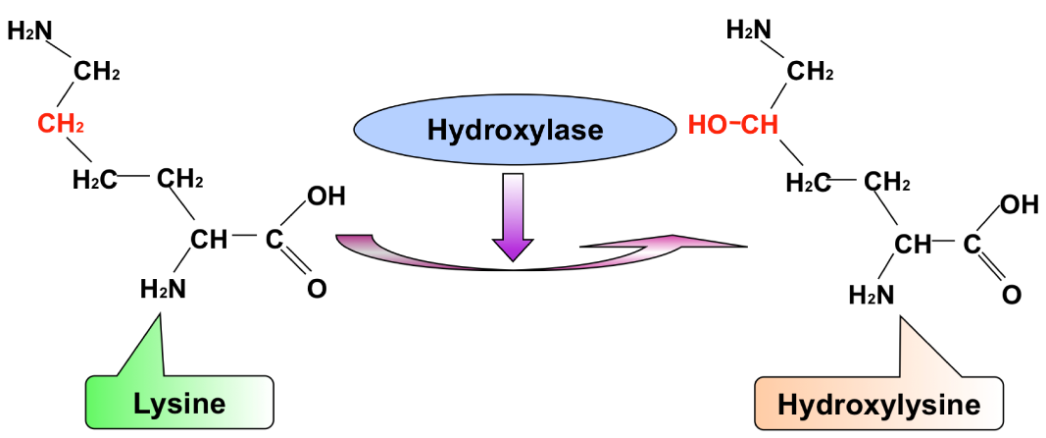
Xu, Y. et al. Int J Mol Sci. 2014.
Figure 1. Schematic Drawing to Show Protein Hydroxylation Occurring at Lysine to Form Hydroxylysine (HyL)
The next critical step in collagen maturation is cross-linking, a process primarily initiated by lysyl oxidase (LOX) enzymes. These enzymes oxidize lysine or hydroxylysine residues into reactive aldehydes, which then undergo condensation reactions to form covalent intermolecular bonds. The dynamics of cross-linking determine whether collagen fibrils acquire lysyl pyridinoline (LP) or hydroxylysyl pyridinoline (HP) linkages, with HP cross-links providing superior stability and longevity. The balance between different types of cross-links reflects tissue-specific demands and adapts to physiological or pathological conditions. Excessive or aberrant cross-linking contributes to tissue stiffness, fibrosis, and altered cellular microenvironments, while insufficient cross-linking compromises tissue strength and resilience.

McKay, T. B. et al. Cells. 2019.
Figure 2. Schematic Depicting Fibrillar Collagen Processing and Crosslinking in Normal, Diabetes Mellitus (DM), and Keratoconus (KC) Microenvironments
Together, collagen lysine hydroxylation and cross-linking represent highly coordinated processes that regulate collagen assembly and mechanical performance. Their precise characterization is indispensable for understanding normal tissue development, unraveling disease mechanisms, and guiding therapeutic strategies in regenerative medicine and biopharmaceutical design.
Analysis Workflow

Sample Submission Suggestions
1. Sample Types
Cell lysates, tissues, biofluids, or purified proteins. For other types, please contact us in advance for tailored preparation guidance.
2. Storage and Shipping
Store at -80°C and ship on dry ice for fresh or lysate samples.
Lyophilized collagen can be shipped at room temperature in moisture-resistant packaging.
Service Advantages
✅ Precision: Site-level characterization using high-resolution mass spectrometry.
✅ Sensitivity: Optimized enrichment methods for low-abundance cross-linked peptides.
✅ Comprehensiveness: Full workflow coverage from sample preparation to functional interpretation.
✅ Customization: Flexible designs to meet project-specific requirements.
✅ Expertise: Experienced team in PTM and collagen biology provides professional data interpretation and technical support.
Applications
1. Connective Tissue Biology
Exploring the role of hydroxylation and cross-linking in fibril formation and tissue mechanics.
2. Fibrosis and Aging Research
Profiling collagen cross-linking abnormalities in fibrotic tissues and age-related modifications that drive tissue stiffening and functional decline.
3. Bone and Cartilage Disorders
Investigating hydroxylation defects in osteogenesis imperfecta and skeletal dysplasias.
4. Cancer Biology
Studying how altered cross-linking shapes the tumor microenvironment and promotes metastasis.
5. Regenerative Medicine
Evaluating collagen scaffolds and biomaterials for tissue engineering applications.
6. Biopharmaceutical Development
Ensuring quality and stability of collagen-based drugs and medical devices.
Our Collagen Lysine Hydroxylation and Cross-linking Analysis Service is designed to provide more rapid, high-throughput, and cost-effective analysis, with exceptional data quality and minimal sample consumption. Free project evaluation, welcome to learn more details.








