Malonylation Proteomics Service
Protein malonylation is a reversible lysine post-translational modification that regulates the charge state, spatial conformation, and biological functions of proteins by adding a malonyl group to lysine residues. This modification is closely associated with various biological processes, including energy metabolism, redox homeostasis, signal transduction, and cellular stress responses. With the advancement of high-resolution mass spectrometry, malonylation proteomics has become an important approach for systematically studying the distribution, functional networks, and dynamic changes of malonylation modifications, and is widely applied in metabolic regulation, biomarker discovery, and functional protein research.
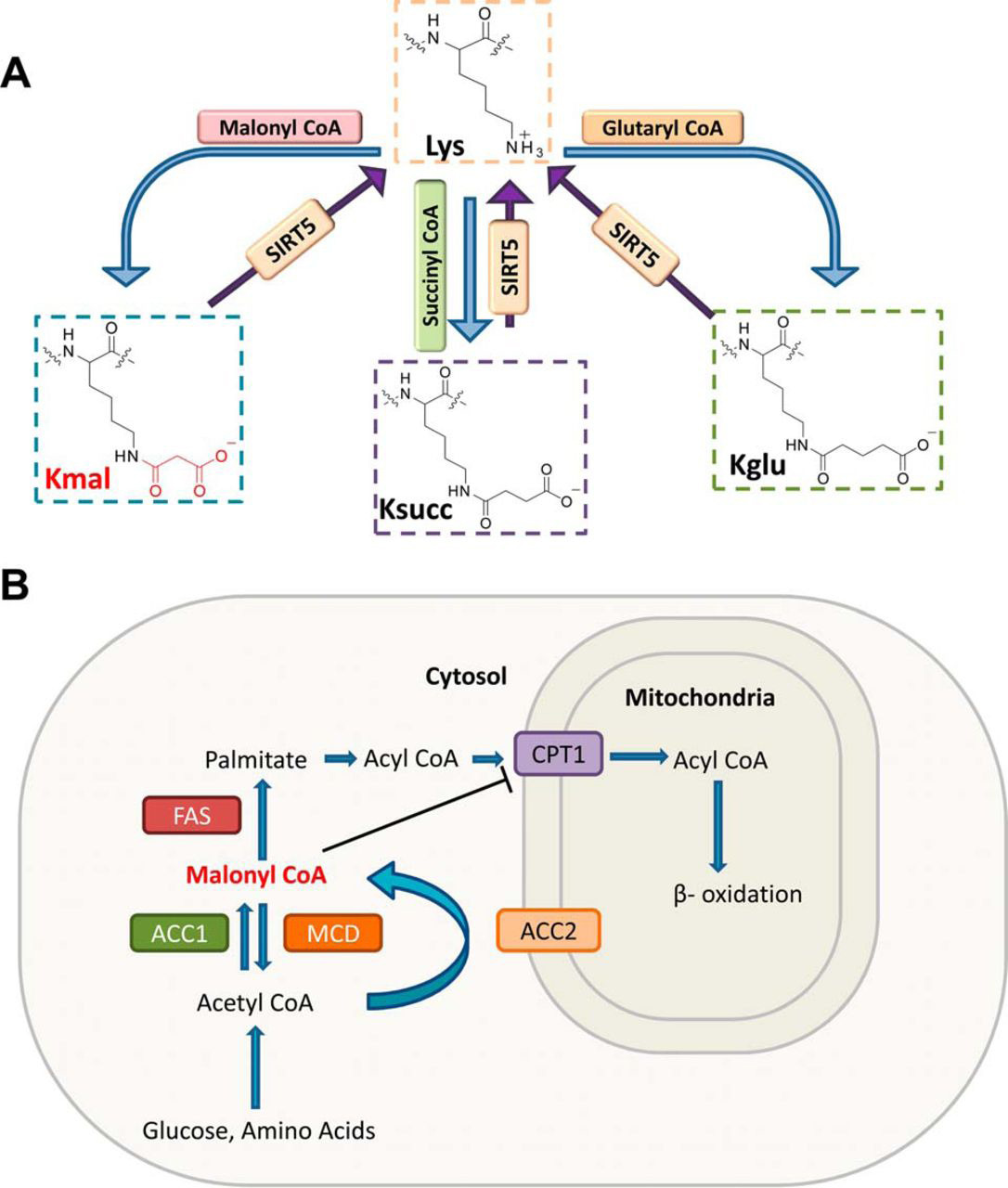
Colak, G. et al. Molecular & Cellular Proteomics, 2015.
Figure 1. Lysine Malonylation and Biosynthesis of Malonyl-CoA.
Services at MtoZ Biolabs
Based on a high-resolution mass spectrometry (LC-MS/MS) platform and efficient peptide enrichment technology, MtoZ Biolabs has launched the malonylation proteomics service to systematically identify and quantitatively analyze protein malonylation modifications. This service achieves precise identification of modification sites and abundance assessment through immuno-enrichment and mass spectrometry detection, and provides information on malonylation site distribution and related proteins through database comparison, offering strong support for functional studies and data interpretation.
Analysis Workflow
1. Protein Extraction and Digestion
Total proteins are extracted from tissue or cell samples and digested with trypsin to obtain peptide mixtures.
2. Malonylated Peptide Enrichment
Malonylated peptides are enriched using specific anti-malonylation antibodies.
3. Mass Spectrometry Detection and Data Acquisition
Peptides are analyzed on a high-resolution LC-MS/MS platform, and fragment spectra are acquired.
4. Data Analysis and Modification Identification
Malonylation sites are identified and quantified through database searching.
5. Functional Annotation and Reporting
Analysis results are integrated, and a standardized report containing functional annotations is generated.
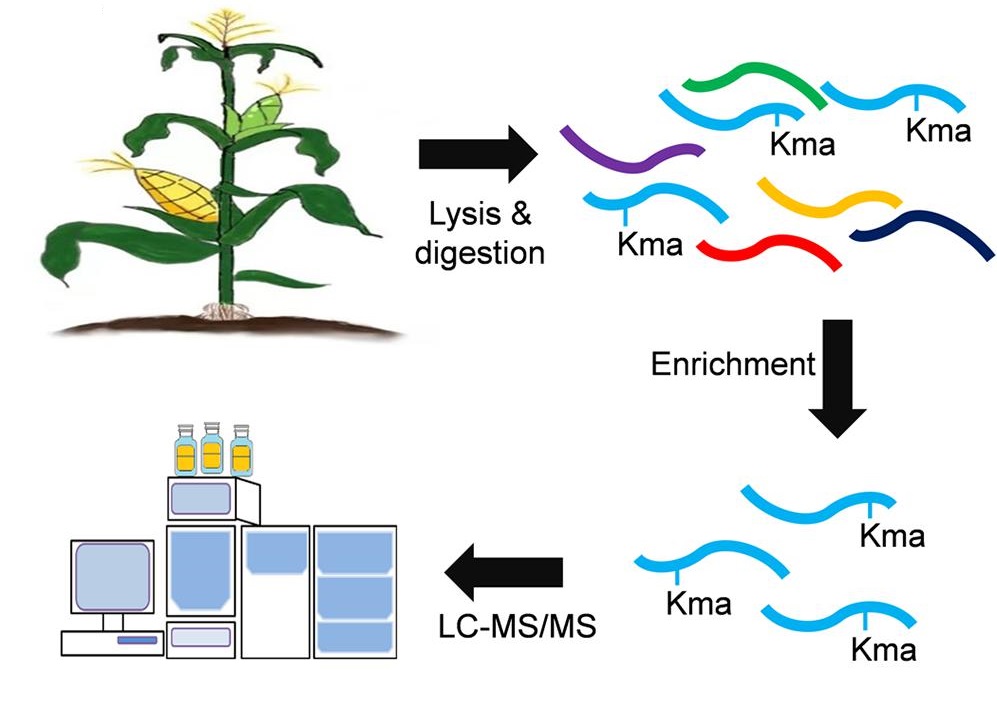
Xu, M. et al. Frontiers in Plant Science, 2021.
Figure 2. Global Identification of Lysine Malonylation Sites in Maize.
Sample Submission Suggestions
1. Sample Types
Cell, tissue, serum, plasma, and microbial samples are acceptable. Protein extracts or lyophilized powders are also supported, making the service suitable for various experimental types and research purposes.
2. Sample Storage and Transportation
Samples should be sealed, protected from light, and stored at -80°C; short-term storage at -20°C is acceptable. Liquid samples should be transported under cold-chain conditions, while lyophilized samples may be shipped at ambient temperature for short durations. Avoid high temperatures, humidity, and repeated freeze-thaw cycles to prevent protein degradation and loss of modifications.
3. Sample Quality Requirements
Samples should maintain good integrity without visible degradation or precipitated impurities. High-salt or surfactant-containing buffers should be avoided to ensure the accuracy and stability of mass spectrometry results.
Service Advantages
1. High-Sensitivity Detection Platform
Powered by a high-resolution LC-MS/MS system, this service enables precise detection and quantitative analysis of low-abundance malonylation modifications.
2. Specific Enrichment Strategy
High-affinity anti-malonylation antibodies are employed to enhance peptide identification efficiency and coverage depth.
3. Standardized Analytical Workflow
A strict quality control system is applied throughout sample preparation and data processing to ensure accuracy and reproducibility.
4. Customized Research Solutions
Analytical strategies are flexibly tailored according to research objectives, accommodating diverse sample types and scientific requirements.
Applications
1. Metabolic Network Research
The malonylation proteomics service can be used to analyze protein malonylation changes under different metabolic conditions.
2. Energy Conversion and Biosynthesis Analysis
By detecting malonylation levels of key enzymes, this service helps evaluate their regulatory roles in fatty acid synthesis, glucose metabolism, and energy balance.
3. Biological Process Regulation Studies
Through systematic identification of malonylated proteins, the service reveals their potential roles in protein complex formation, metabolic pathways, and cellular function regulation.
4. Functional Protein Characterization
The malonylation proteomics service enables exploration of how malonylation modification influences protein structure and activity.
FAQ
Q1: Can Multiple Samples Be Analyzed Simultaneously for Malonylation Differences?
A1: Yes. MtoZ Biolabs provides quantitative strategies based on TMT or Label-Free approaches to compare malonylation levels among samples under different treatments or physiological states, thereby revealing modification trends.
Q2: Is Malonylation Easily Confused with Acetylation?
A2: No. MtoZ Biolabs uses high-resolution LC-MS/MS combined with specific antibody enrichment strategies to distinguish between characteristic ions and molecular mass differences of the two modifications, ensuring accurate results.
Q3: Can Malonylation Analysis Be Integrated with Other PTM Proteomics Studies?
A3: Yes. MtoZ Biolabs supports integrated analyses combining malonylation proteomics with acetylation, phosphorylation, ubiquitination, and other modification proteomics to construct a comprehensive protein modification network.







