Cysteine Redoxome Proteomics Analysis Service
Based on a high-resolution mass spectrometry (LC-MS/MS) platform and highly selective chemical labeling techniques, MtoZ Biolabs has launched the cysteine redoxome proteomics analysis service which enables systematic analysis of the redox modification states of cysteine residues in proteins. This service integrates differential labeling, enrichment and purification, and quantitative mass spectrometry detection to achieve precise identification and abundance assessment of modified sites. Through data comparison and functional annotation, researchers can obtain information on the distribution patterns, dynamic changes, and potential regulatory networks of protein redox modifications, providing reliable data support for oxidative stress research and cellular homeostasis regulation.
Overview
Cysteine redoxome proteomics is a scientific field that investigates redox modifications of cysteine residues in proteins, such as disulfide bond formation, S-nitrosylation, S-sulfonation, and S-glutathionylation. These modifications play critical roles in regulating protein activity, conformational stability, and signal transduction, serving as key mechanisms for cellular responses to oxidative stress and the maintenance of homeostasis. By systematically analyzing the modification states and dynamic variations of cysteine residues, researchers can reveal their roles in redox regulation, metabolic modulation, and functional protein studies.
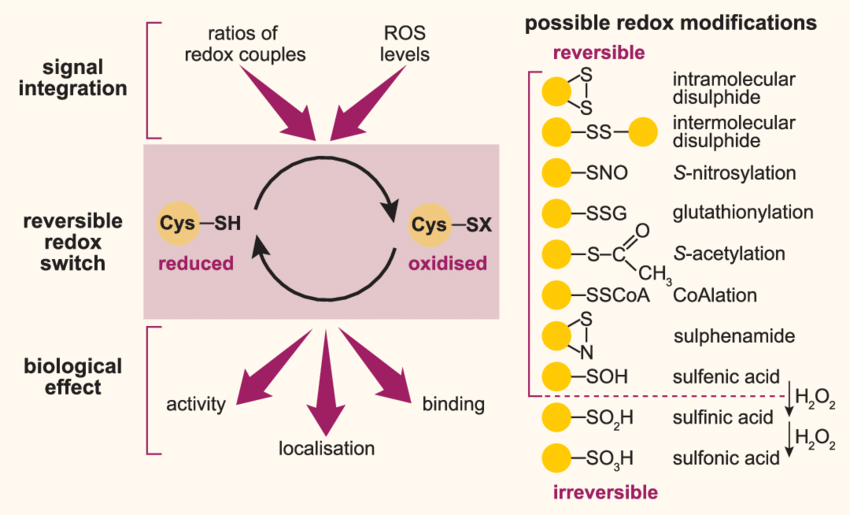
Lennicke, C. et al. Biochemical Society Transactions, 2020.
Figure 1. Scheme of Redox Signalling and Possible Cysteine-Based Redox Modifications.
Analysis Workflow
1. Protein Extraction and Processing
Total proteins are extracted from samples, and cysteine redox modification states are stabilized through chemical derivatization or differential labeling.
2. Digestion and Peptide Enrichment
After proteolytic digestion, specific labeling and enrichment strategies are applied to selectively capture peptides containing cysteine modifications.
3. Mass Spectrometry Detection and Data Acquisition
High-resolution LC-MS/MS is used to detect peptide signals and obtain fragmentation spectra of redox modifications.
4. Data Analysis and Modification Identification
Database searching and algorithmic analysis are performed to identify cysteine modification sites, modification types, and abundance variations.
5. Functional Annotation and Report Output
Modification information is integrated for pathway and functional enrichment analysis, and a standardized result report is generated.
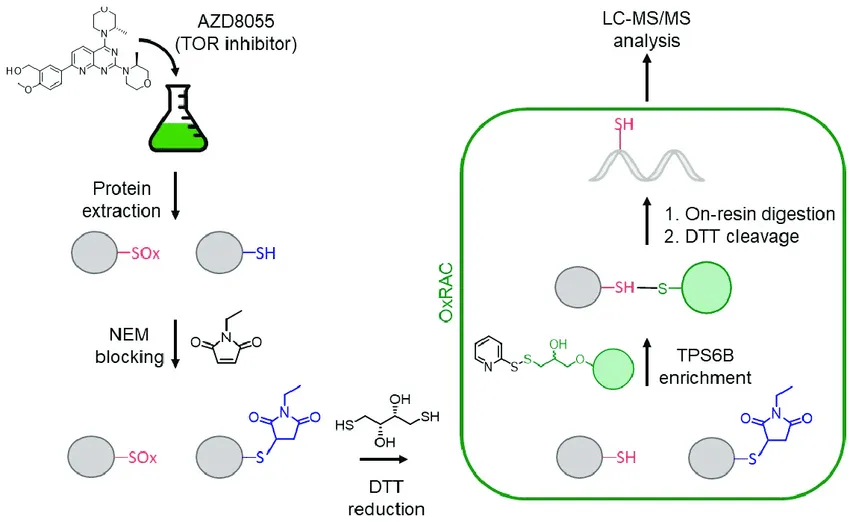
Ford, M M. et al. Cells, 2019.
Figure 2. Example Workflow of Cysteine Redox Proteomics.
Sample Submission Suggestions
1. Sample Type
Cells, tissues, serum, plasma, and microbial samples are acceptable. Protein extracts or lyophilized samples are also supported. It is recommended to avoid strong oxidizing or reducing agents during sample processing to maintain the native cysteine modification state.
2. Sample Storage
Samples should be sealed, protected from light, and stored at -80°C for long-term preservation or at -20°C for short-term storage. Repeated freeze-thaw cycles should be avoided.
3. Sample Transportation
Liquid samples should be transported under cold-chain conditions, while lyophilized samples may be shipped at room temperature for short periods. Avoid exposure to high temperatures, humidity, and direct light to ensure sample stability and analytical accuracy.
Service Advantages
1. High-Resolution Detection Capability
Powered by an advanced LC-MS/MS platform, this service enables precise detection and quantitative analysis of cysteine redox modifications.
2. Multi-Level Modification Identification
Capable of simultaneously identifying multiple redox forms, including disulfide bonding, sulfenylation, sulfinylation, and glutathionylation.
3. Standardized Analytical Workflow
A rigorous quality control system is implemented from sample preparation to data interpretation, ensuring accuracy and reproducibility of results.
4. Customized Research Solutions
Analytical strategies can be flexibly tailored according to research objectives and sample characteristics to meet diverse experimental needs.
Applications
1. Redox State Monitoring
Cysteine Redoxome Proteomics Analysis Service can be used to evaluate the dynamic changes of cysteine modifications under different physiological or treatment conditions.
2. Metabolic and Energy Regulation Research
By analyzing redox modifications of key metabolic enzymes, this service helps reveal their roles in energy conversion and metabolic homeostasis.
3. Protein Structure and Function Studies
Identification of cysteine modification sites allows assessment of their impact on protein conformational stability and catalytic activity.
4. Environmental Stress Response Analysis
Cysteine Redoxome Proteomics Analysis Service can be applied to detect protein oxidative modifications induced by oxidative stress, metal ions, or chemical treatments.
FAQ
Q1: What Types of Modifications Can Cysteine Redoxome Proteomics Analysis Detect?
A1: It can detect multiple cysteine redox modification forms, including disulfide bonds (-S-S-), sulfenylation (-SOH), sulfinylation (-SO₂H), sulfonylation (-SO₃H), and glutathionylation (-SSG).
Q2: Does Sample Handling Affect the Redox State?
A2: Yes. Exposure to air, strong oxidants, or repeated freeze–thaw cycles may cause modification changes. Therefore, MtoZ Biolabs recommends handling samples at low temperatures, protected from light, and under antioxidant conditions.
Q3: Can Quantitative Analysis Be Performed?
A3: Yes. By combining TMT or label-free quantification strategies, the changes in cysteine modification levels among different treatment groups can be compared.








