Glycopeptide Enrichment Service
MtoZ Biolabs provides Glycopeptide Enrichment Service through a comprehensive portfolio of strategies including multilectin affinity chromatography, hydrophilic interaction chromatography, metal oxide affinity chromatography, immobilized metal affinity chromatography, and hydrazide chemistry. By selecting or combining these methods according to project goals and glycan types of interest, we deliver highly efficient enrichment workflows fully compatible with LC-MS/MS platforms. Our tailored solutions ensure broad glycopeptide coverage, reliable structural characterization, and reproducible quantitative data to support both discovery research and pharmaceutical development.
Overview
Glycopeptides are proteolytic fragments that retain covalently attached glycans, serving as direct links between peptide backbones and glycan structures. They are invaluable targets in glycoproteomics because they provide site-specific information on protein glycosylation. However, their low abundance and structural heterogeneity present major analytical challenges. Without enrichment, glycopeptides are often masked by the overwhelming presence of non-glycosylated peptides in mass spectrometry analysis.
To address this limitation, several enrichment strategies have been developed, each exploiting unique chemical or structural features of glycans:
1. Multilectin Affinity Chromatography (M-LAC)
By combining multiple lectins with different carbohydrate-binding specificities, M-LAC enables the simultaneous enrichment of a broad range of glycopeptides. This approach is suitable for both N- and O-glycopeptides and is especially useful when comprehensive glycoproteome coverage is required.
2. Hydrophilic Interaction Chromatography (HILIC)
Owing to the strong hydrophilicity of glycans, HILIC selectively enriches glycopeptides from complex mixtures. It is one of the most widely used methods for N-glycoproteomics, offering robust performance and high reproducibility.
3. Metal Oxide Affinity Chromatography (MOAC)
Utilizing transition metal oxides such as titanium dioxide, MOAC captures negatively charged glycans, particularly sialylated glycopeptides. It enhances the detection of acidic glycan species that may otherwise be underrepresented.
4. Immobilized Metal Affinity Chromatography (IMAC)
Similar to MOAC, IMAC relies on chelated transition metal ions (e.g., Fe³⁺, Ga³⁺, Ti⁴⁺, Zr⁴⁺) to bind negatively charged groups. IMAC provides complementary selectivity to MOAC and is often employed to target sialylated glycopeptides.
5. Hydrazide Chemistry
This strategy involves oxidizing sialic acid residues to generate aldehydes, which are then covalently captured by hydrazide groups. It is particularly effective for enriching sialylated glycopeptides and facilitates selective analysis of glycoproteins with terminal sialic acid residues.
Each of these methods offers distinct advantages, and their complementary nature allows for combined application to maximize glycopeptide recovery and improve coverage. By integrating appropriate enrichment strategies, researchers can significantly enhance the sensitivity, specificity, and depth of glycoproteomic analysis, thereby gaining more reliable insights into biological processes and disease mechanisms.
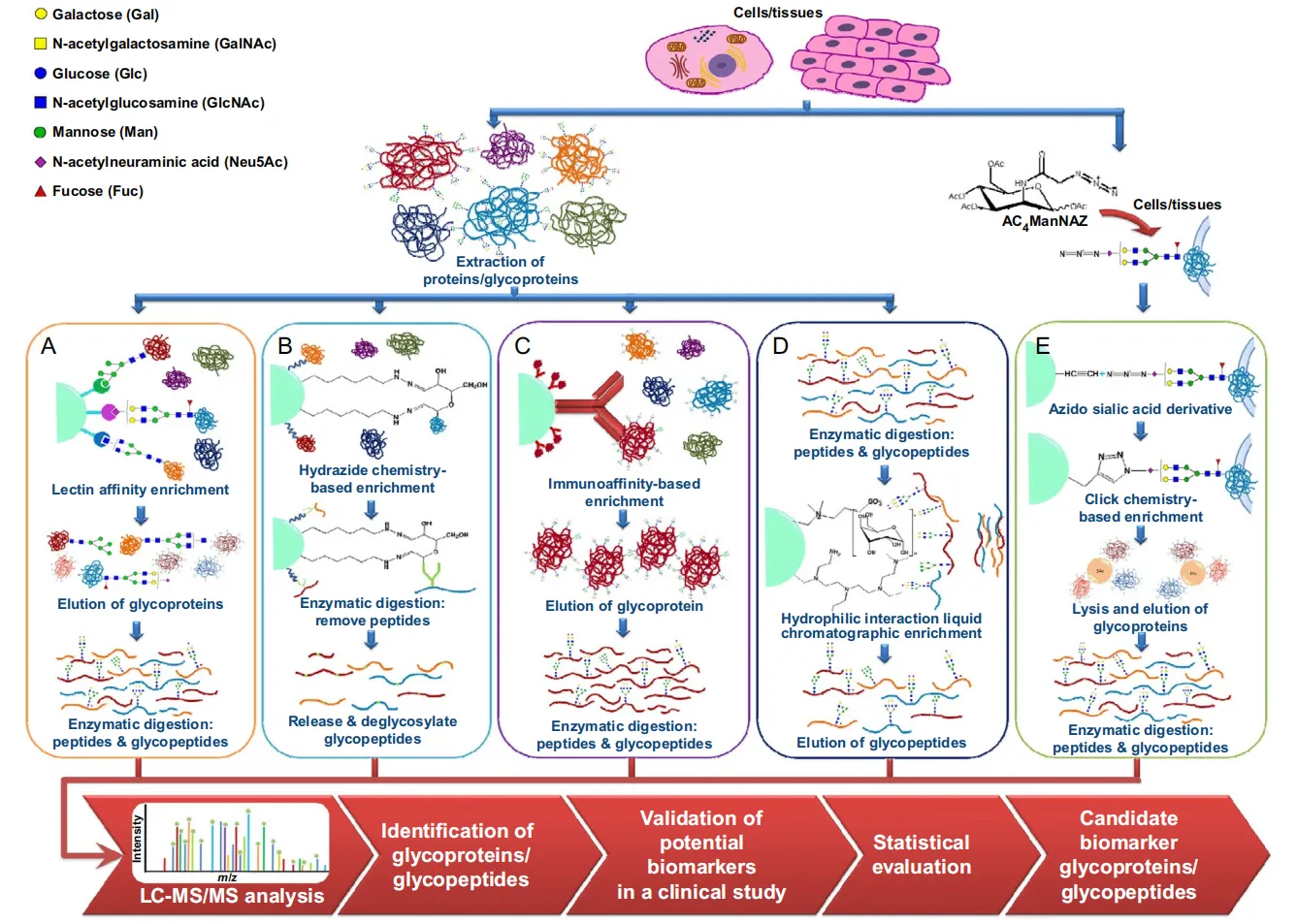
Zhu R. et al. Methods Enzymol. 2017.
Figure 1. Typical Glycoproteomics Workflow.
Service Advantages
Some advantages of Glycopeptide Enrichment Service are as follows:
1. Comprehensive Strategy Portfolio
MtoZ Biolabs offers multiple enrichment approaches, including multilectin affinity chromatography, HILIC, MOAC, IMAC, and hydrazide chemistry. This broad portfolio enables researchers to tailor enrichment workflows to different glycan types and research objectives.
2. Enhanced Sensitivity and Coverage
By selectively capturing low-abundance glycopeptides and reducing interference from non-glycosylated peptides, our service significantly improves glycoproteome depth and structural resolution.
3. Customized and Flexible Workflows
We design enrichment protocols according to sample type, glycan characteristics, and project goals, ensuring optimal recovery whether the focus is on N-glycopeptides, O-glycopeptides, or sialylated species.
3. Compatibility with High-Resolution MS
All enrichment workflows are optimized for LC-MS/MS analysis, ensuring accurate mass detection, reliable glycan assignment, and reproducible quantification.
Sample Submission Suggestions
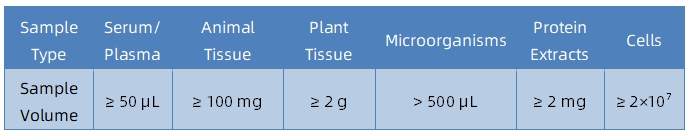
It is recommended to contact our technical support team before submitting samples to determine sample suitability and obtain tailored submission guidelines.
Applications
Examples of applications of Glycopeptide Enrichment Service are as follows:
1. Comprehensive Glycoproteome Profiling
Enrichment strategies increase the relative abundance of glycopeptides, allowing in-depth mapping of glycosylation across diverse biological systems.
2. Biomarker Discovery and Validation
Enriched glycopeptides from clinical samples such as serum or plasma facilitate the identification of glycosylation-based biomarkers with diagnostic and prognostic potential.
3. Therapeutic Protein Development
Glycopeptide enrichment ensures precise characterization of glycosylation in monoclonal antibodies and biotherapeutics, supporting quality control, regulatory submissions, and biosimilar development.
4. Environmental and Microbial Studies
Enriched glycopeptides from microbial proteins provide insights into host–environment interactions and glycosylation mechanisms in microbial adaptation.
FAQ
Q1: Which Enrichment Strategy Should I Choose for my Project?
A1: The choice depends on your research objectives and glycan types of interest. For example, HILIC is often applied to N-glycopeptides, M-LAC is suitable for both N- and O-glycopeptides, while MOAC and IMAC are effective for sialylated species. Our experts will design customized workflows tailored to your samples and goals.
Q2: How Reproducible Are the Results?
A2: MtoZ Biolabs applies standardized protocols, rigorous quality control, and advanced instrumentation to ensure reproducibility. Our workflows are validated across multiple sample types and research contexts.








