Targeted Glycosylation Profiling Service
MtoZ Biolabs provides comprehensive Targeted Glycosylation Profiling Services designed to accurately characterize glycan structures, linkage types, and site-specific occupancy in client-specified proteins. Using state-of-the-art LC-MS/MS platforms and glycopeptide enrichment strategies, we offer high-resolution identification and quantification of glycosylation patterns to support protein characterization, therapeutic development, and quality assurance across research and industrial applications.
What Is Glycosylation?
Protein glycosylation is a crucial post-translational modification (PTM) in which carbohydrate chains, known as glycans, are covalently attached to specific amino acid residues within a protein. This modification occurs primarily in the endoplasmic reticulum and Golgi apparatus and affects a wide range of biological processes, including protein folding, stability, transport, and cell communication.
1. Composition and Structure of Glycans
Protein-linked glycans are composed of repeating monosaccharide units such as glucose, galactose, mannose, fucose, N-acetylglucosamine (GlcNAc), and N-acetylneuraminic acid (sialic acid). These monosaccharides can be linked together through various glycosidic bonds to form complex branched or linear structures.
2. Types of Protein Glycosylation
🔸N-Linked Glycosylation: Glycans are attached to the amide nitrogen of asparagine residues within the consensus sequence Asn-X-Ser/Thr.
🔸O-Linked Glycosylation: Glycans are linked to the hydroxyl groups of serine or threonine residues.
3. Biological Significance of Glycosylation
🔸Structural Stability: Glycans help maintain protein conformation and protect against proteolysis.
🔸Functional Regulation: They influence ligand binding, enzyme activity, and immune recognition.
🔸Quality Control: Glycosylation acts as a checkpoint during protein maturation and secretion.
🔸Disease Association: Aberrant glycosylation is often correlated with tumor progression, inflammation, and congenital disorders.
By analyzing the composition, linkage, and site-specific distribution of these glycans, researchers can gain essential insights into protein function, molecular interactions, and therapeutic quality control.
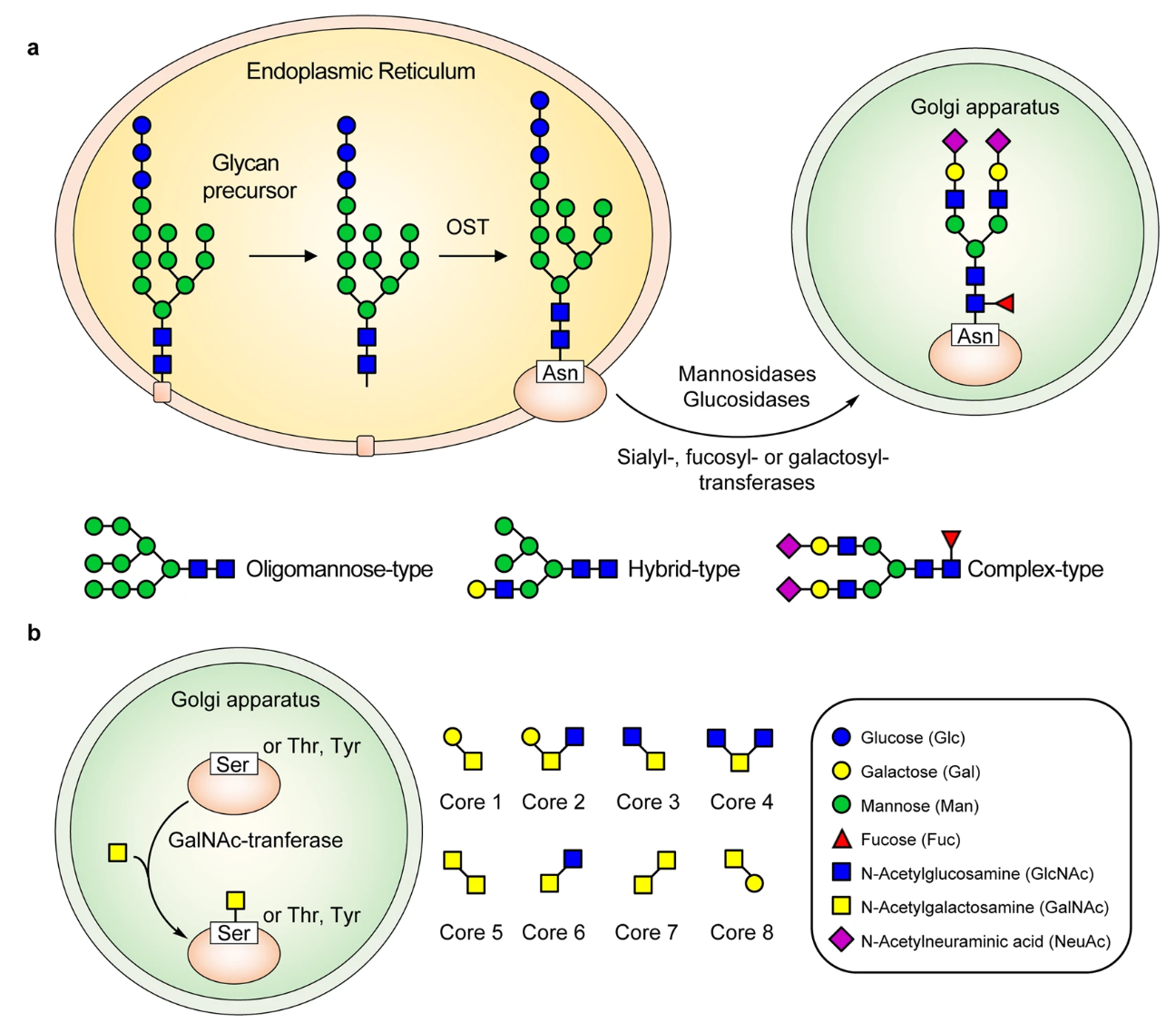
Gong, Y. et al. Signal Transduct Target Ther. 2021.
Figure 1. The Formation Process of N-Glycosylation and O-Glycosylation in Protein
Targeted Glycosylation Profiling Service at MtoZ Biolabs
MtoZ Biolabs offers a full suite of Targeted Glycosylation Profiling Services focusing on specific target proteins or defined glycosylation types, providing both qualitative and quantitative insights.
💠Targeted Glycosylation Site Mapping
Identify and localize N-linked and O-linked glycosylation sites on the specified protein with peptide-level precision using optimized digestion and high-resolution MS analysis.
💠Glycan Composition Analysis
Determine the types and relative abundances of monosaccharides (e.g., mannose, galactose, fucose, GlcNAc, sialic acid) that make up the glycans attached to the protein.
💠Glycosidic Linkage Determination
Characterize the linkage types (α/β and 1→2, 1→3, 1→4, 1→6) and branching patterns of glycans using LC-MS/MS fragmentation and exoglycosidase sequencing.
💠Site-Specific Glycosylation Quantification
Quantify glycan occupancy and relative abundance at each glycosylation site to assess structural microheterogeneity and lot-to-lot consistency.
💠Glycosylation Structural Determination
Elucidate the detailed glycan structure, including branching and terminal modifications, combining LC-MS/MS, NMR, and FTIR for comprehensive profiling.
💠Glucose Unit (GU) Value Determination
Calculate GU values based on HILIC retention times using glucose oligomer standards, providing standardized parameters for glycan identification and cross-lab comparison.
Each analysis is target-specific and can be customized according to the client's protein, research objective, and regulatory requirements, ensuring accurate and reproducible glycosylation characterization.
Workflow of Targeted Glycosylation Profiling Service
1. Protein Preparation and Enzymatic Digestion
Samples are enzymatically digested under optimized conditions to generate glycopeptides while maintaining glycan integrity.
2. Glycopeptide Enrichment
Enrichment methods such as HILIC, PGC, or lectin affinity capture are applied to selectively isolate glycosylated peptides from complex mixtures.
3. High-Resolution LC-MS/MS Analysis
Glycopeptides are analyzed using Orbitrap or Q-TOF mass spectrometers coupled with nanoLC systems. MS/MS fragmentation (HCD, ETD, or EThcD) enables simultaneous identification of peptide backbones and attached glycans.
4. Data Processing and Quantification
Advanced bioinformatics tools interpret MS data, identify glycosylation sites, determine glycan compositions, and quantify site occupancy across different samples.
Why Choose MtoZ Biolabs?
☑️High Specificity: Focused analysis of glycosylation on designated target proteins with site-level accuracy.
☑️Comprehensive Structural Insight: Integrates glycan composition, linkage, and structural determination for complete characterization.
☑️Customizable Workflow: Analytical methods tailored to each protein type and project requirement.
☑️Expert Interpretation: Data analyzed by experienced scientists to deliver clear, meaningful, and actionable results.
☑️Quality and Reproducibility: Standardized procedures ensure consistent, validated results suitable for regulatory or publication use.
Applications of Targeted Glycosylation Profiling Service
1. Therapeutic Protein Characterization
Assess glycosylation sites, glycan types, and structural integrity of therapeutic proteins such as monoclonal antibodies, fusion proteins, and recombinant enzymes to ensure proper folding and bioactivity.
2. Antibody Quality Evaluation
Verify Fc-region glycosylation patterns in IgG subclasses that influence effector functions, such as antibody-dependent cellular cytotoxicity (ADCC) and complement activation.
3. Stability and Formulation Assessment
Monitor glycosylation stability during manufacturing, purification, and long-term storage to identify structural changes that may impact potency or shelf life.
4. Disease-Associated Protein Research
Investigate altered glycosylation of specific target proteins implicated in cancer, immune disorders, or neurodegenerative diseases to elucidate functional mechanisms.
FAQ
Q1: What Types of Samples Are Suitable?
A1: We accept a wide range of protein and biological samples, including purified proteins, recombinant proteins, antibodies, and other therapeutic proteins. Cell lysates, tissues, and biological fluids can also be submitted, with MtoZ Biolabs performing target protein extraction and preparation.
Q2: How Should I Prepare My Samples?
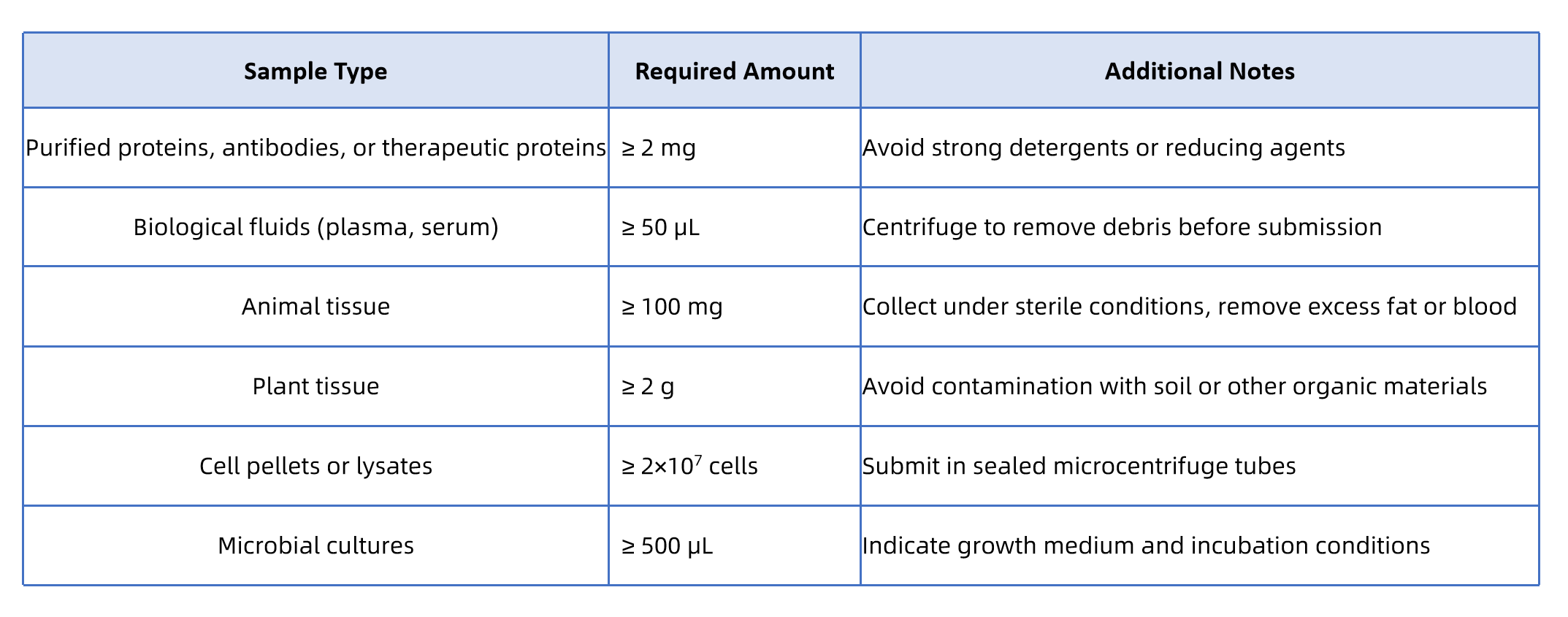
- All samples should be stored at –80°C.
- Use dry ice or ice packs during shipment to preserve protein integrity.
- Avoid freeze–thaw cycles that may alter disulfide linkages.
For more information, please refer to Sample Submission Guidelines for Proteomics.
Q3: What Is the Service General Workflow?
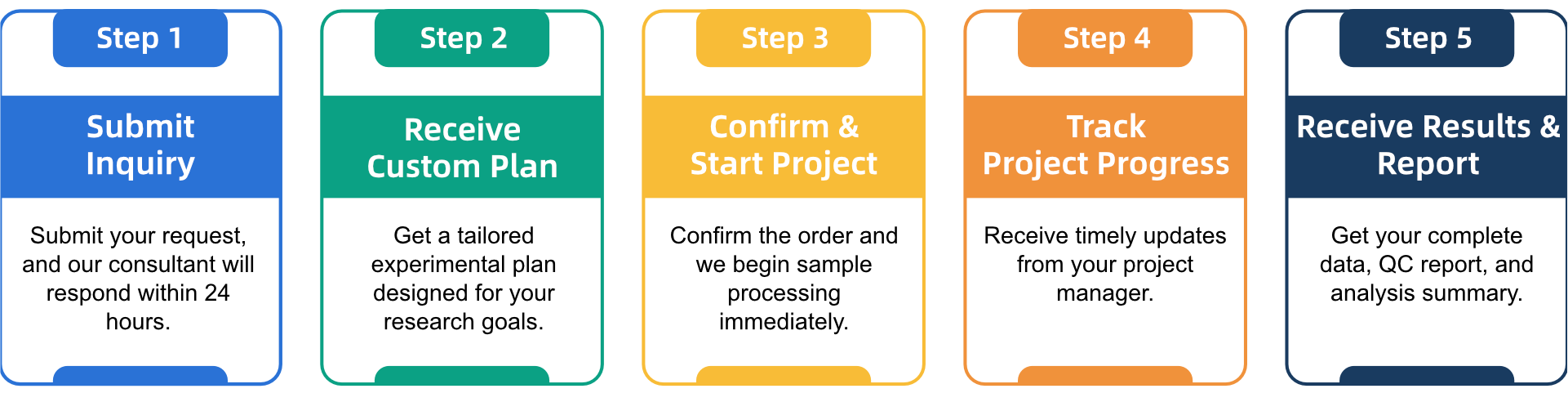
Q4: What Data Formats Are Provided?
Results are delivered in standard and easy-to-access formats, including:
- Raw MS data files (.raw, .wiff, .d)
- Processed data and quantification tables (.xlsx, .csv)
- Spectral and chromatogram images (.pdf, .png)
- Final analytical report (.pdf)
All deliverables are compatible with common data analysis software. Additional file formats or data structures can be provided upon request to meet specific research or publication requirements.
Start Your Project with MtoZ Biolabs
Contact us to discuss your experimental design or request a quote. Whether for structural verification, biopharmaceutical quality assessment, or biological function studies, our targeted glycosylation profiling platform enables confident decision-making backed by high-resolution data.








