Venom N-Glycan Analysis Service
MtoZ Biolabs provides Venom N-Glycan Analysis Service to deliver high-resolution qualitative and quantitative profiling of venom-derived N-glycans. By integrating optimized enzymatic release protocols, advanced HILIC-UHPLC MS and MALDI-TOF MS platforms, and expert bioinformatics pipelines, we enable accurate identification, and quantification of glycans in diverse venom samples. Our tailored workflows ensure reliable insights for applications ranging from toxin mechanism studies and antivenom development to biomarker discovery and biopharmaceutical research.
Overview
Venom proteins are a rich source of biologically active molecules with diverse therapeutic and toxicological potential. Many venom components are glycoproteins, and their N-glycans profoundly influence protein folding, stability, receptor recognition, and immunogenicity. Because venom N-glycans often display unique structural features, including unusual branching patterns and species-specific modifications, their characterization is essential for understanding toxin function and guiding biomedical applications. Advances in mass spectrometry and chromatographic separation have enabled high-resolution profiling of venom N-glycans, allowing researchers to identify glycan structures, quantify their abundance, and explore their roles in venom activity, disease mechanisms, and antivenom development.
Technical Principles
The principle of venom N-glycan analysis lies in characterizing the oligosaccharide structures covalently attached to venom proteins at specific asparagine residues. Enzymatic cleavage methods, such as treatment with PNGase F or PNGase A, release intact N-glycans from glycoproteins without disturbing their native architecture. Once liberated, glycans can be derivatized to enhance detection sensitivity and then analyzed using high-resolution mass spectrometry platforms such as MALDI-TOF MS or LC-MS coupled with hydrophilic interaction chromatography. By interpreting precise mass-to-charge ratios and fragmentation patterns, researchers can determine glycan composition, branching, and linkage features. Integration with curated glycan databases allows mapping of structural diversity and heterogeneity.
Analysis Workflow
The general analytical workflow for Venom N-Glycan Analysis Service is as follows:
1. Sample Preparation
Venom proteins are extracted, purified, and prepared under optimized conditions to preserve glycan structures.
2. Enzymatic Glycan Release
N-linked glycans are released using PNGase F or PNGase A to detach glycans from protein backbones.
3. Glycan Purification and Labeling
Released glycans are purified to remove interfering components and derivatized with fluorescent reagents such as 2-aminobenzamide (2-AB) for enhanced detection.
4. Chromatographic Separation
HILIC-UHPLC is employed to resolve complex glycan mixtures by polarity and structure, ensuring improved analysis of venom N-glycans.
5. Mass Spectrometry Analysis
MALDI-TOF MS or LC-MS provides detailed information on glycan mass, composition, and isomeric diversity with high sensitivity.
6. Data processing and reporting
The bioinformatics process integrates spectral data with the glycan database to generate a comprehensive report, including glycan profiles, quantitative results, and so on.
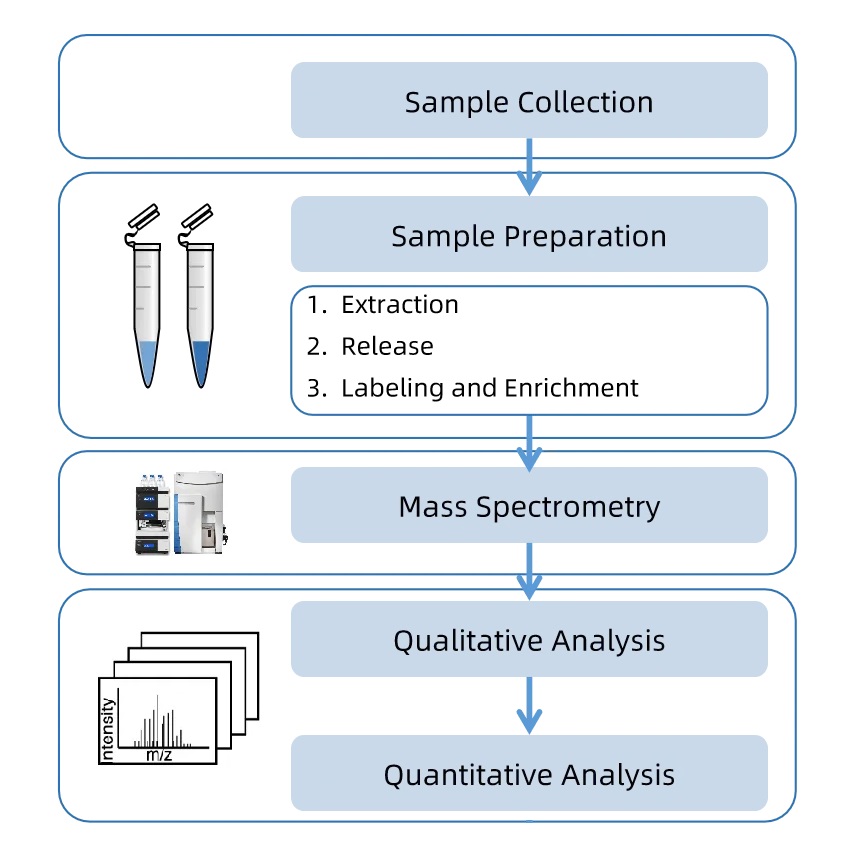
Figure 1. N-Glycan Analysis Service Workflow
Service Advantages
1. High Sensitivity and Specificity
MALDI-TOF MS and HILIC-UHPLC MS platforms deliver accurate detection of low-abundance glycans.
2. Comprehensive Profiling
Venom N-Glycan Analysis Service enables both qualitative and quantitative analysis for a complete glycan landscape.
3. High-Data-Quality
Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all N-Glycan Analysis data, providing clients with a comprehensive data report.
4. Customized Workflows
Flexible protocols designed to meet diverse venom research needs.
Sample Submission Suggestions
1. Sample Types
Crude venom, purified venom proteins, or venom gland extracts.
2. Storage
Store samples at -80°C; avoid repeated freeze-thaw cycles.
3. Shipping
Ship samples on dry ice in sealed, labeled containers.
It is recommended to contact our technical support team before submitting samples to determine sample suitability and obtain tailored submission guidelines.
Applications
Application examples for Venom N-Glycan Analysis Service:
1. Toxin Mechanism Studies
Reveal how glycosylation affects venom protein activity, stability, and interaction with cellular receptors.
2. Antivenom Development
Characterize venom glycoproteins to support the design of effective and targeted antivenom therapies.
3. Drug Discovery and Biopharmaceuticals
Identify venom-derived glycoproteins as potential leads for therapeutic development.
4. Comparative Venom Research
Explore glycosylation diversity across species, uncovering evolutionary adaptations in venom composition.








