MS-Based Viral Glycoproteomics Service
Viral glycoproteomics aims to systematically study the types, structural characteristics, and distribution patterns of glycosylation modifications in viral glycoproteins. As a key post-translational modification, glycosylation plays an important role in the folding, assembly, and stability of viral proteins and affects the recognition and interaction between viruses and their hosts. By systematically analyzing the glycan composition and modification patterns of viral glycoproteins, researchers can gain deeper insights into their structural features and functional differences, which are widely applied in virology, structural biology, immunology, and biopharmaceutical research.
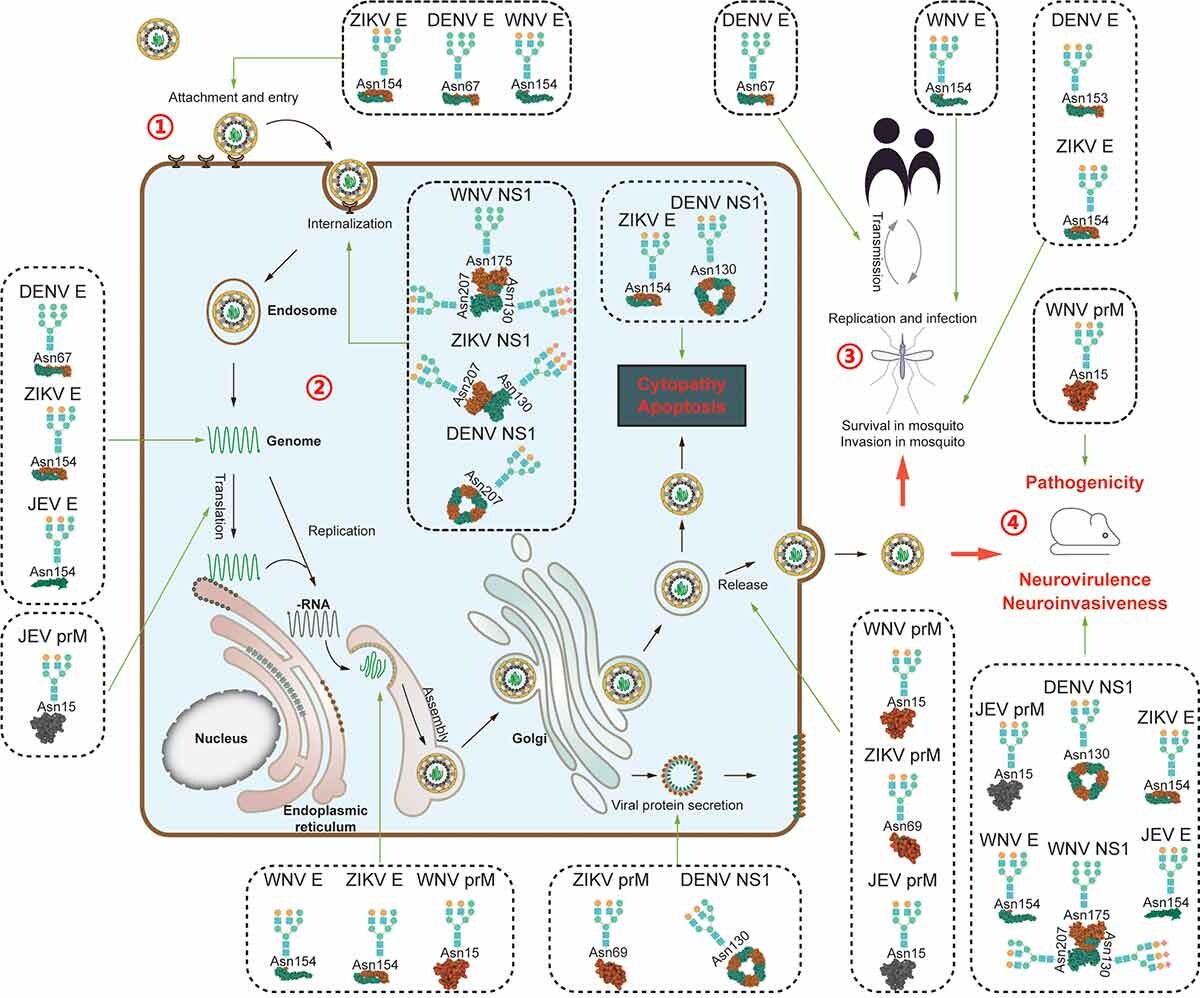
Feng, T T. et al. Virulence, 2022.
Figure 1. Diverse Functions of Glycosylation of Viral Proteins from Mosquito-Borne Flaviviruses.
Services at MtoZ Biolabs
Based on advanced mass spectrometry analysis platforms, MtoZ Biolabs has launched the MS-based viral glycoproteomics service which enables systematic identification and quantitative analysis of glycosylation modifications in viral glycoproteins. This service integrates multiple high-resolution mass spectrometry techniques, including LC-MS/MS, MALDI-TOF-MS, and Orbitrap-MS, combined with glycopeptide enrichment, enzymatic deglycosylation, and glycan structure characterization strategies. It comprehensively characterizes glycan types, linkage patterns, and modification sites of glycoproteins from multiple dimensions, providing valuable data support for viral research.
Analysis Workflow
1. Protein Extraction and Digestion
Total proteins are extracted from viral or infected samples and enzymatically digested to obtain peptide mixtures.
2. Glycopeptide Enrichment
Lectin affinity, HILIC, or enzymatic deglycosylation strategies are used to enrich glycopeptides.
3. Mass Spectrometry Detection
High-resolution detection of glycopeptides is performed using LC-MS/MS, MALDI-TOF-MS, or Orbitrap-MS to acquire fragment spectra.
4. Data Analysis
Database searching and algorithmic analysis are used to identify glycosylation sites, glycan types, and structural characteristics.
5. Data Interpretation and Report Generation
Quantitative and visual analyses of glycoprotein modification features are performed, and a standardized analytical report is generated.
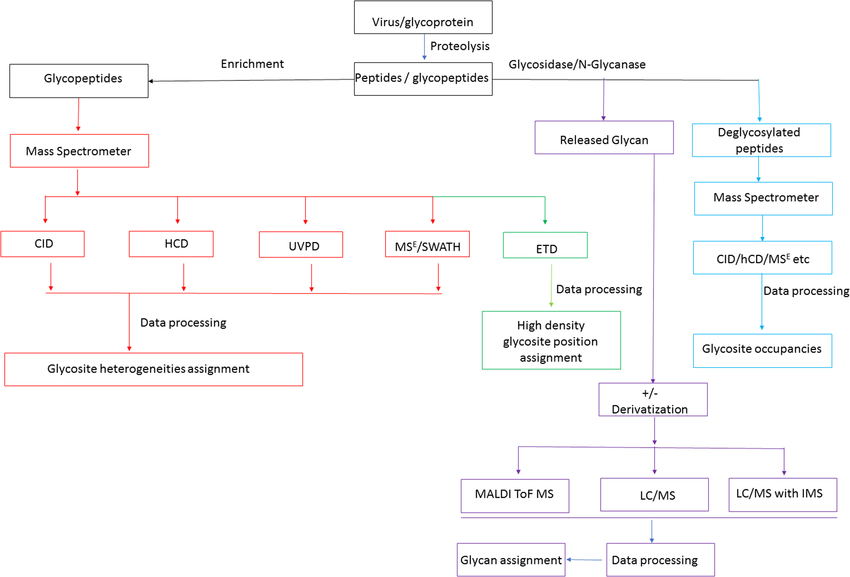
Cipollo, J F. et al. Mass Spectrometry Reviews, 2020.
Figure 2. General Glycomics Workflow for Virus Analysis with Multiple Options Shown.
Sample Submission Suggestions
1. Sample Type
Purified viral samples, infected cell lysates, or viral protein extracts are acceptable. Freeze-dried powders, solutions, and tissue samples are also supported, suitable for various viral sources.
2. Sample Storage
Samples should be sealed and stored at −80°C for long-term preservation or at −20°C for short-term use. Repeated freeze-thaw cycles should be avoided to prevent protein degradation and glycan structure damage.
3. Sample Transportation
Sealed, moisture-proof packaging is recommended. Liquid samples should be shipped under cold-chain conditions, while freeze-dried samples can be sent at ambient temperature for short durations, avoiding high temperature and humidity.
Service Advantages
1. High-Resolution Mass Spectrometry Platforms
Equipped with Orbitrap, Q Exactive, and MALDI-TOF systems to achieve highly sensitive detection and precise identification of glycopeptides.
2. Multi-Level Enrichment Strategies
Utilizes Lectin affinity, HILIC, and enzymatic deglycosylation methods to improve glycopeptide coverage and identification depth.
3. Standardized Analytical Workflow
Implements rigorous quality control throughout the process to ensure result stability and reproducibility.
4. Customized Research Support
Provides flexible analytical design tailored to viral types and specific research objectives.
Applications
1. Host Interaction Studies
The MS-based viral glycoproteomics service can be used to evaluate the binding characteristics between viral glycoproteins and host receptors or immune molecules.
2. Infection Process Monitoring
By comparing glycosylation changes at different stages of infection, it helps in understanding infection dynamics.
3. Vaccine and Antibody Research
The MS-based viral glycoproteomics service can be applied to assess glycosylation features of candidate vaccine proteins or antibody recognition sites.
4. Biomarker Discovery
By analyzing glycoprotein modification differences related to infection, it provides valuable data for identifying potential biomarkers.
FAQ
Q1: Can Mass Spectrometry Distinguish Different Types of Glycosylation Modifications?
A1: Yes. Based on a high-resolution LC-MS/MS platform combined with glycan databases and fragment ion analysis, it can accurately differentiate N-glycans, O-glycans, and their various branching structures.
Q2: Can Glycosylation Differences between Samples Be Compared?
A2: Yes. We provide relative quantification analysis to evaluate glycosylation variations across viral strains, hosts, or treatment conditions.
Q3: How Is the Accuracy of Glycan Structure Identification Ensured?
A3: We use multi-stage fragmentation (MS/MS and MS³) along with database matching and manual validation to ensure high-confidence structural annotation.
Q4: Is Deglycosylation or Enzymatic Digestion Required before MS Analysis?
A4: Generally, yes. Enzymatic digestion generates glycopeptide fragments suitable for MS detection, and in some cases, PNGase F or O-glycosidase treatment is applied to assist in site localization.








